

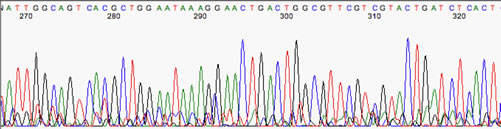
Use only ABI (PN: N8010560) plates which you may obtain from us. Submit samples in 8-strip PCR tubes or 96-well plates. For example, if your sample name is AB123 and the primer is M13F the electropherogram file name will be AB123-M13F.ab1. The file name of your data will consist of your sample name and primer. Drop off your samples and request forms in the refrigerator at the front of the lab.
Print out 2 copies of your request-one for yourself and one to submit to the lab. hairpin or GC rich) next to the samples in question when you fill out the web request form. We will use extra polymerase, blend polymerases and / or alter the thermocycling conditions if you select “difficult template” and also indicate the nature of the difficulty (e.g. The LIMS asks if the template is “difficult to sequence”. Your account number must be exactly seven digits with no spaces, dashes etc. Once logged in, follow the instructions and call if you have any problems. Requests are submitted to:įirst time users must create an account before logging into the LIMS.
Also see the Sample Submission in Detail section for additional information. Templates are mixed with primers and submitted in 12 ul as described in the Template Primer Quantity Table section.
We use a laboratory information management system (dnaLIMS) to handle orders and distribute data. Template Preparation for Successful Automated DNA Sequencing How to Order Large DNA (>30,000bp) BACs, PACs, YACs, cosmids, fosmids Templateįor the following templates submit sample and primer in SEPARATE tubes: Template/Primer should be submitted in THE SAME tube. If your sample and primer combined is less than 12ul add deioinzed water to bring it up to 12ul. Recommended template and primer concentrations in a 12ul sample. 40-45☌.) If Tm is low, make the primer longer.


 0 kommentar(er)
0 kommentar(er)
